


Menu
Triantafyllidis Research Group



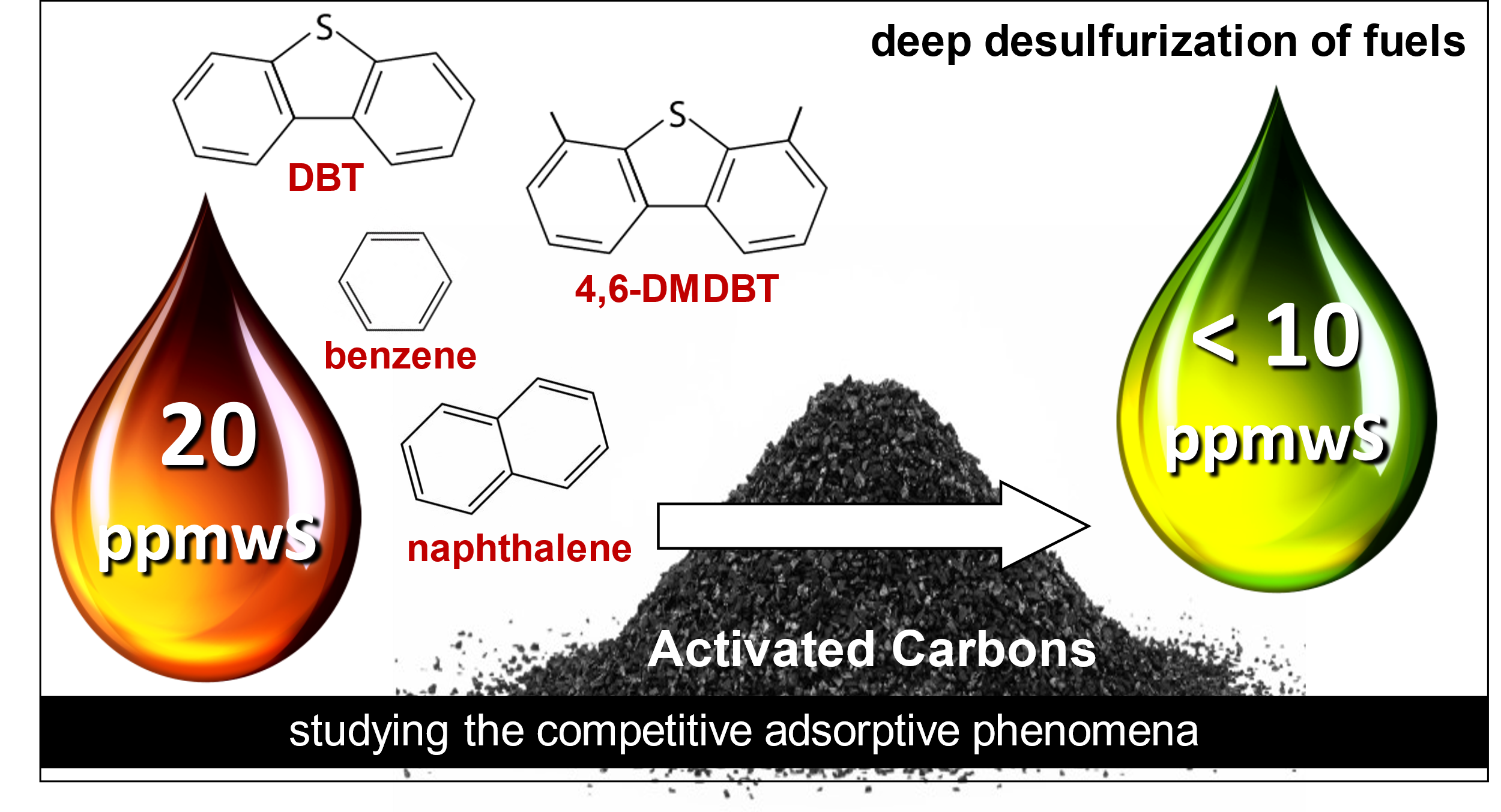
Check out our new paper on the deep desulfurization of model fuels by metal-free activated carbons.
Our article entitled: “Deep desulfurization of model fuels by metal-free activated carbons: The impact of surface oxidation and antagonistic effects by mono- and poly-aromatics ” has been recently published in the Journal of Molecular Liquids. https://doi.org/10.1016/j.molliq.2022.118661
Abstract
The main novel objective of this work is the evaluation of the adsorptive “deep” desulfurization efficiency under ambient conditions and studying in detail the effects arisen from the presence of competitors as in real fuels. Towards this direction, several activated carbons (commercially available and chemically modified/oxidized counterparts) were examined using a (diesel) model fuel containing initially 20 ppmwS of 4,6-dimethyldibenzothiophene (4,6-DMDBT) and 20 ppmwS of dibenzothiophene (DBT), as well as mono- and di-aromatic competitors, namely benzene and naphthalene, in hexadecane. To gain better insights regarding the adsorption sites, the involved mechanisms, and the impact/antagonistic effect arisen by the presence of the aromatics in high concentration, simpler model fuels were also utilized while emphasis was also given on exploring which physicochemical features, textural (N2 adsorption) or surface chemistry (Boehm titration and surface pH measurements), play a key role. In all cases, the oxidation treatment enhanced the adsorption capacity of the carbons, despite a decrease on the porosity, by introducing additional surface functional groups, indicating that surface chemistry plays a vital role in adsorptive desulfurization and that difference in chemical nature adsorption sites exists. The best performing oxidized carbon (SX PLUS-ox) showed a 67.7% 4,6-DMDBT removal (4.0 mgS/g capacity) in pure hexadecane, while the addition/co-presence of the aromatics led to slightly lower desulfurization of 57.8% (3.6 mgS/g), revealing the low antagonistic effect due to the aromatics. When DBT was also present, the 4,6-DMDBT removal was 51.2% (3.5 mgS/g) while the DBT removal 50.9 % (3.6 mgS/g), leading to a high total thiophenic adsorption capacity of 7.1 mgS/g. Even the addition of the mono- and di-aromatics with concentrations as in real diesel fuel, the best performing carbons after oxidation demonstrated a superior desulfurization performance, since more than 10 ppmwS can still be removed from the model fuel. The results designate that the oxidation of porous activated carbon can be assumed as an effectual materials design strategy towards adsorptive deep desulfurization, since different oxygen containing surface function groups that can act as adsorption sites were created and have a crucial selectivity against thiophenes compared to the aromatics.
Read full text here. (https://doi.org/10.1016/j.molliq.2022.118661)