


Menu
Triantafyllidis Research Group



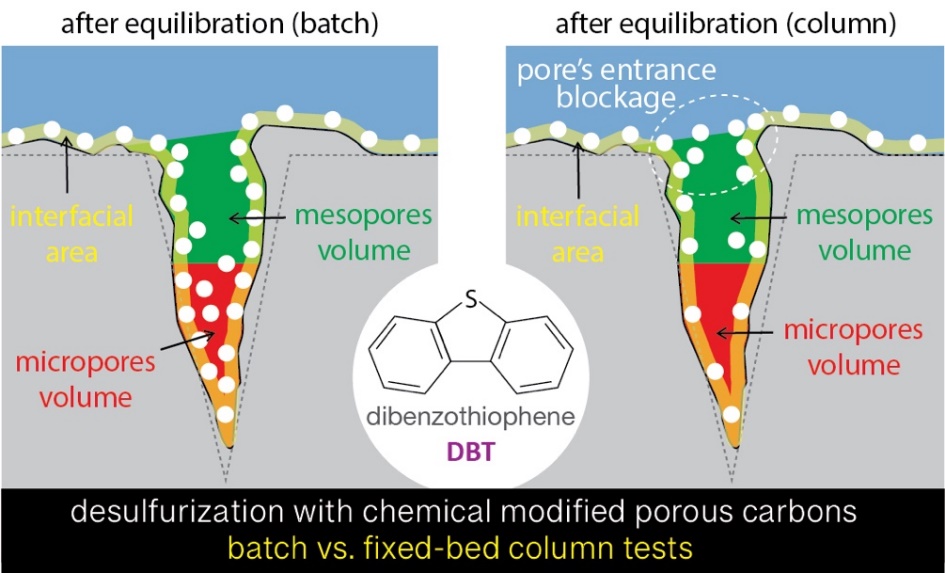
Check out our recent publication on deep desulfurization in dynamic and batch mode over a commercial and a chemically modified activated porous carbon.
Our latest work regarding the efficiency for deep desulfurization (removal of dibenzothiophene, DBT) of a benchmark commercial activated porous carbon (BAX) and its chemically modified counterparts both in dynamic (fixed-bed column) and batch experiments was recently published in the Journal of Colloids and Surfaces A: Physicochemical and Engineering Aspects with the title “Dynamic/column tests for dibenzothiophene (DBT) removal using chemically functionalized carbons: Exploring the effect of physicochemical features and breakthrough modeling”.
Abstract
The efficiency for deep desulfurization (removal of dibenzothiophene, DBT) of a benchmark commercial activated porous carbon (BAX) and its chemically modified counterparts was evaluated both by dynamic (fixed-bed column) and batch experiments. Oxidation of BAX led to alteration of various key physicochemical features, namely textural parameters and surface chemistry heterogeneity. These alterations affected controversially the desulfurization efficiency/capacity, since the effect was positive for batch tests but slightly negative for column tests. The oxidized sample BAX-O4 was the only one capable to decrease the initial concentration of sulfur from 20 to less than 10 ppmwS at the batch experiments. In case of the dynamic tests, even though a slightly faster exhaustion was observed for the oxidized samples, the adsorption kinetics were faster. It was also shown that oxidation of BAX resulted in denser column packing. Thus, a higher amount of adsorbent material per unit volume (bed density) can be achieved which will lead to increment of the column’s desulfurization efficiency. Analysis of the breakthrough data by curve modeling showed that carbon-beds operated smoothly under equilibrium conditions. By detailed analysis and comparisons of the physicochemical features of the initial and exhausted samples as well as considering the fitting of the adsorption results at various kinetic models, it was feasible to determine the key removal mechanisms and the involved interfacial phenomena occurring during the adsorption.
To read the full text, click here. (https://doi.org/10.1016/j.colsurfa.2022.128597)