


Menu
Triantafyllidis Research Group



21/11/2022
Just published!
Our new article entitled: “Modeling the Liquid Fuel Desulfurization Efficiency of Activated Carbons before and after Chemical Treatment: The Competitive Role of Mono- and Diaromatics” was just published in the Journal of Industrial & Engineering Chemistry Research.
Abstract
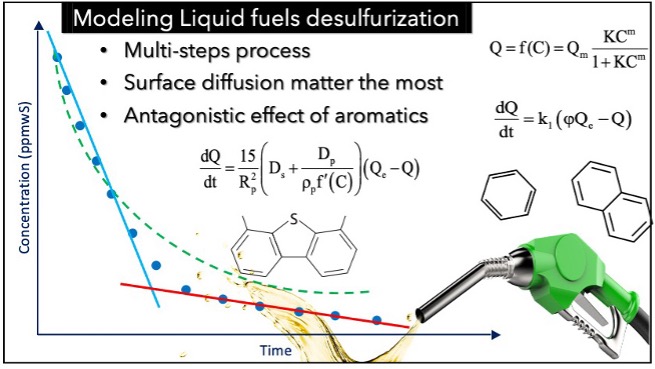
The scope of this work is the experimental and modeling study of adsorptive deep desulfurization of model fuels utilizing activated carbons. Emphasis is given on how oxidative chemical treatment affects the textural and surface chemistry properties and hence the desulfurization kinetics and performance, while the effect of the copresence of aromatics is also investigated. The derivation of the kinetic model and the understanding of adsorption mechanism(s) are also among the main focuses of the work. Two model solutions, one to mimic gasoline/petrol (DBT in hexane) and another for diesel (4,6-DMDBT in hexadecane), with low initial sulfur concentration (20 ppmwS), as well as a model fuel consisting of 4,6-DMDBT, benzene, and naphthalene in hexadecane mimicking the real diesel fuel, were prepared. Adsorption equilibrium and kinetic experiments were conducted, while the results were fitted by simple empirical models and phenomenological models. Chemical treatment through oxidation enhanced the performance of the carbon, reaching a final concentration of less than 2 ppmwS in the case of DBT in hexane and 7.2 ppmwS for 4,6-DMDBT in hexadecane, thus achieving deep desulfurization. In contrast, the copresence of aromatic compounds in high concentrations decreased the efficiency of the carbons, revealing their competitive character in adsorptive desulfurization. Surface diffusion is considered the main mechanism governing the adsorption kinetics. A strong inhibition in diffusion is observed due to the competitive adsorption/interactions, as well as of the already adsorbed compounds. Interestingly, as regards the DBT/hexane system, the hindrance is so strong that the model has to be replaced by a system of two steps in series having kinetics of quite disparate time scales.
Read full text here. (https://pubs.acs.org/doi/full/10.1021/acs.iecr.2c02794)